For localized information and support, would you like to switch to your country-specific website for {0}?
Transforming molecular diagnostics and screening for every setting and situation
Redefining molecular testing with comprehensive and scalable solutions
Roche molecular lab diagnostic solutions enable disease management across the healthcare continuum, from screening and prevention to diagnosis and monitoring. High-performance PCR systems, an expansive menu of molecular assays, and advanced digital solutions help you accomplish:
- Centralized end-to-end automation with pre- and post-analytics
- Lower volume or specialized testing, including syndromic testing and blood and plasma screening
- Point of Care diagnostics
Roche was an early adopter of PCR technology, leading the way in real-world applications for screening, diagnosing, and monitoring infectious diseases. Today, Roche offers a comprehensive menu of molecular tests for use in various lab settings, situations, and clinical areas including infectious diseases, cervical health, sexual health, respiratory, transplant, and oncology.
Innovation solutions for healthcare decision support
At Roche, we continuously advance our offerings across platforms and menus to transform traditional molecular labs. We aim to simplify systems and increase efficiency to improve the user experience and ensure labs can deliver timely, trustworthy results.
Roche molecular lab solutions advance patient care across the entire continuum: from scientific discovery to screening, prevention, clinical diagnosis, and monitoring. Rooted in our commitment to innovation, our portfolio of solutions helps ensure access to testing across different settings for confident healthcare decisions.
Shaping the future of molecular labs
At Roche, we push boundaries to fuel innovation that streamlines molecular testing for timely answers that our customers can trust.
Experience a new level of molecular lab efficiency with the Molecular Work Area from Roche.
Solutions designed to help transform your research ideas into clinically viable tests.
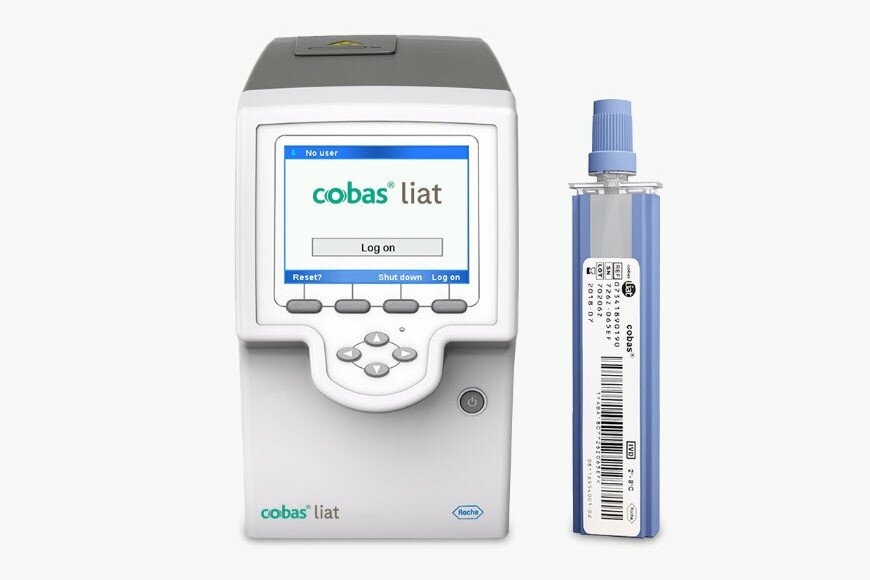
Gold standard PCR technology at the Point of Care for near patient testing in primary care and hospital settings.
Benefits of molecular lab solutions from Roche
Optimize lab workflows with sample-to-answer solutions
- Automate molecular workflows across lab settings with digital and physical solutions and an extensive test menu
- Simplify testing by consolidating various molecular tests onto fewer systems from a single manufacturer, reducing fragmentation and complexity
- Reduce manual steps by eliminating hands-on tasks to minimize errors and free up staff for higher-value activities
- Leverage digital lab solutions to integrate data streams, improve operational efficiency, and derive lab insights
- Implement load-and-go workflows that require no pre-sorting
A broad range of flexible solutions for your laboratory needs
- Ensure efficient PCR testing across diverse settings from centralized laboratories to Point of Care settings
- Accommodate complex test menus and intervene easily when a sample requires prioritization with intelligent automation
- Bridge research and clinical diagnostics by leveraging open platforms and channels
- Integrate testing across disciplines into efficient workflows
Push boundaries through innovation
- Embrace future opportunities and stay ahead of future challenges with Roche's unique combination of cutting-edge solutions
- Leverage Roche’s extensive experience as pioneers and leaders in PCR technology since the 1990s
- Drive your laboratory innovations through open platforms and channels
- Go beyond operational efficiency with navify® digital solutions for laboratory insights
For your lab, choose a partnership built on trust
- Rely on a globally recognized market leader with thousands of systems installed worldwide1
- Access and benefit from world-class support through Roche Diagnostics consulting, implementation, and service solutions, including our Medical Affairs, Training, Market Access teams, Roche Healthcare Consulting, and more
- Support sustainability with a company ranked among the top 3 most sustainable healthcare companies in the Dow Jones Sustainability Indices for 15 years2
Roche pre-analytical system transforms molecular workflows for Vall d Hebron lab
Related health topics
References:
- Roche Diagnostics Ltd. Data on file.
- PHARMIWEB.JOBS. Roche named among top three most sustainable healthcare companies in the Dow Jones Sustainability Indices [Internet; cited 2024 Jun 7] Available from: https://www.pharmiweb.jobs/article/roche-named-among-top-three-most-sustainable-healthcare-companies-in-the-dow-jones-sustainability-indices